 | |||||||||||
Image Gallery Pollen Grain, Mouse Intestine, Mouse Kidney, Heart Muscle Ca,
UPGRADES
Applications:
Two-photon excitation fluorescence microscopy offers several advantages over confocal laser scanning microscopy (CLSM), such as the use of less damaging NIR wavelengthss, reduced scattering, excellent optical sectioning capabilities even in dense and thick samples, elimination of pin holes, higher light collection efficiency, excitation and photobleaching limited to the focal region. However, in common with CLSM, the image rate is still quite low. Multifocal Multiphoton Microscopy: To speed up the image rate, researchers have used polygon mirrors, resonant scanners, and mirolens arrays. The problems with the microlens array design are low light throughput, non-uniform illumination, lens aberrations, interfoci cross-talk and limited field of view. Please refer to the publication for a comparison of the axial resolution of practical Nipkow-disk confocal fluorescence microscopy with that of multifocal multiphoton microscopy. TriMScope: LaVision BioTec’s TriMScope is based on a patented beamsplitter that splits up an incoming laser beam into up to 64 beamlets which are scanned simultaneously in the object plane. This results in either 64 times brighter images or 64 times higher image rates compared to standard single beam multiphoton scanning microscopes. The foci in the object plane are aligned in a single line and the number of foci can be easily switched from 64 to 32, 16, 8, 4 and to a single beam. Image rates up to 3500 Hz are possible. The TriMScope beam divider is a compact and easy-to-use device that utilizes exclusively flat optics for dividing the incoming beam with high light efficiency, avoiding aberrations and producing equally intense foci in the sample. As the beamlets are inherently shifted with respect to each other by several picoseconds, there is no cross-talk. Spatial and axial resolutions are diffraction limited. In addition, single line excitation allows convenient coupling to the input slit of an imaging spectrograph yielding real-time spectral sectioning in the x-y and x-z planes.
Upgrade 3D FLIM: TriMScope in conjunction with the ultrahigh rep. rate, picosecond gated PicoStar HR (200ps gate width @ 110 MHz rep. rate) camera allows real-time 3D FLIM. Upgrade FRAP: An additional laser can be coupled in the optical path which can be scanned via the XY scanner and used for FRAP studies or uncaging. ACCESSORIES: Various accessories for microscopy, imaging and spectroscopy are available. PUBLICATIONS: Time-resolved fluorescence imaging of solvent interactions in microfluidic devices Richard K. P. Benninger, Oliver Hofmann, James McGinty, Jose Requejo-Isidro, Ian Munro, Mark A. A. Neil, Andrew J. deMello and Paul M. W. French OPTICS EXPRESS 2005, 13(16), 6275-6285 Fluorescence Imaging of Two-Photon Linear Dichroism: Cholesterol Depletion disrupts Molecular Orientation in Cell Membranes Richard K P Benninger, Bjorn Onfelt, Mark AA Neil, Daniel M Davis and Paul MW French Biophysical Journal 2005, 88:609-622 Two-dimensional imaging without scanning by multifocal multiphoton microscopy Comparison of the axial resolution of practical Nipkow-disk confocal fluorescence microscopy with that of multifocal multiphoton microscopy:theory and experiment Fluorescence-lifetime imaging with a multifocal two-photon microscope Multiphoton multifocal microscopy exploiting a diffractive optical element Opt Lett. 2003, 28(20):1918-20 Sacconi L, Froner E, Antolini R, Taghizadeh MR, Choudhury A, Pavone FS Fluorescence resonance energy transfer analysis of protein-protein interactions in single living cells by multifocal multiphoton microscopy Majoul I, Straub M, Duden R, Hell SW, Soling HD J Biotechnol. 2002 Jan;82(3):267-77 Space-multiplexed multifocal nonlinear microscopy Hell SW, Andresen V J Microsc. 2001 Jun;202(Pt 3):457-63 Live cell imaging by multifocal multiphoton microscopy Straub M, Lodemann P, Holroyd P, Jahn R, Hell SW. Eur J Cell Biol. 2000 Oct;79(10):726-34 Time multiplexing and parallelization in multifocal multiphoton microscopy Egner A, Hell SW J Opt Soc Am A Opt Image Sci Vis. 2000 Jul;17(7):1192-201 | |||||||||||


 Live Cell Imaging, high speed Ca++ imaging, vesicle trafficking, Brain, nervous system, Kidney, Tissue Imaging Modes time lapse, Z-Stack, Spectral, 3D-FLIM, FRET, FRAP, SHG, Anisotropy.......
Live Cell Imaging, high speed Ca++ imaging, vesicle trafficking, Brain, nervous system, Kidney, Tissue Imaging Modes time lapse, Z-Stack, Spectral, 3D-FLIM, FRET, FRAP, SHG, Anisotropy....... 
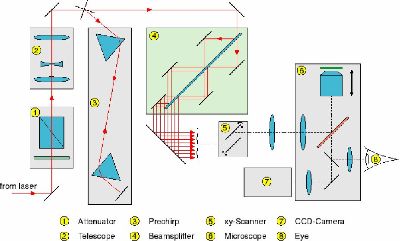 1. Attenuator 2. Telescope, 3. Prechirp, 4. Beam Splitter, 5. XY Scanner, 6. Microscope, 7. CCD camera, 8. Eyepiece
1. Attenuator 2. Telescope, 3. Prechirp, 4. Beam Splitter, 5. XY Scanner, 6. Microscope, 7. CCD camera, 8. Eyepiece